Answer:
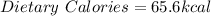
Step-by-step explanation:
Hello!
In this case, since we can see that the heat released by the combustion of the mayonnaise is absorbed by the bomb calorimeter and water, we can set up the following equation:
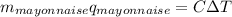
Whereas q for mayonnaise stands for the heat due to its usage; thus, we plug in to obtain:
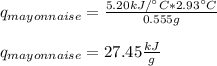
Now, if 10.0 g are eaten, the energy provided by it turns out:
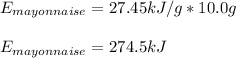
And we need that value in dietary calories, 1 kcal:
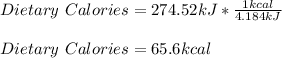
Best regards!