The problem can be solved using Ideal Gas Equation.
The equation is given below.
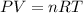
Step 1 : Calculate moles of the gas (n)
Where P = pressure of the gas in atm = 3.05 atm
V = Volume of the gas in Liters = 10.0 L
n = number of moles of the gas
R = Gas constant = 0.08206 L-atm/mol-K
T = Temperature of the gas in Kelvin
To convert T from Celsius to Kelvin unit, we add 273
Hence we have T = 24 + 273 = 297 K
Let us plug in the above values in ideal gas equation
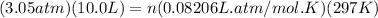
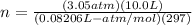 ... Unit K gets cancelled
... Unit K gets cancelled
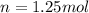
Number of moles of gas are 1.25
Step 2 : Find molar mass using mol value
Mole and molar mass can be related to each other by the following expression
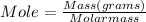
The mass of the gas is given as 25.25 g
moles (n) = 1.25 mol
After plugging the values we get,
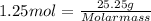
Molar mass =
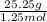
Molar mass =
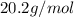
Molar mass of the gas is 20.2 /mol
Step 3 : Find identity of the gas using periodic table
From the periodic table , we can see that noble gas Neon has molar mass 20.18g/mol .
Hence the given gas is Neon which is represented by the symbol is "Ne"