Given mass of Na is 23 g
Volume of water =
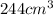
Mass of water =
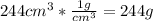
Total solution mass = 23 g + 244 g = 267 g
Specific heat capacity of water = 4.18 J/K.g
Equation relating mass, heat, specific heat capacity and temperature change is:
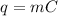 ΔT
ΔT
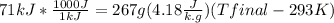
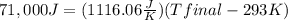
Tfinal = 356.6 K
Therefore, the final temperature of water will be 356.6 K