Answer:- Boiling point of the given solution is 100.3 degree C.
Solution:- The equation for solving this type of boiling point elevation problems is, delta Tb = i x m x kb
Where, delta Tb is the elevation in boiling point
i is Van't hoff factor and it's theoretical value is the number of ions an ionic compound gives. For example, NaCl gives total two ions(one sodium and one chloride ion).
m is molality and kb is the molal elevation constant. For water, kb is 0.512.
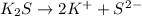
It gives two potassium ions and one sulfide ion. So, i = 2+1 = 3
let's plug in the values in the equation.
delta Tb = 3 x 0.195 x 0.512
delta Tb = 0.3
pure water boils at 100 degree C. So, the boiling point of the solution = 100 + 0.3 = 100.3 degree C