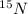 :
:
Atomic number of Nitrogen-15= Number of protons = 7
Mass number = Number of protons + Number of neutrons = 15
Number of neutrons = 15 - 7 = 8
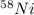 :
:
Atomic number of Nickel-58= Number of protons = 28
Mass number = Number of protons + Number of neutrons = 58
Number of neutrons = 58 - 28 = 30
The number of neutrons in N-15 is 8 and in Ni-58 is 30