Hello!
The molarity of the HBr solution is 0,172 M.
Why?
The neutralization reaction between LiOH and HBr is the following:
HBr(aq) + LiOH(aq) → LiBr(aq) + H₂O(l)
To solve this exercise, we are going to apply the common titration equation:
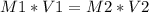
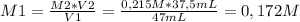
Have a nice day!