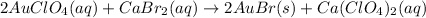Answer:-
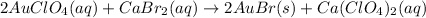
Explanations:- It's a double replacement reaction where a precipitate of silver(I)bromide is formed. A dpuble replacement reaction in general looks as AB + CD \rightarrow AD + CB
To balance the equation we need to multiply gold compounds on both sides by 2 and the balanced equation is..