Answer : The correct answer for atomic fraction of Zn is 4.37
Following are the steps to find atomic fraction of Zinc in Zn-Al alloy :
Step 1 : Find mass of Zn in alloy .
Given : wt%( weight % ) of Zn = 10%
Lets assume complete mass of alloy is 100g ,so the mass of Zn =>
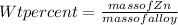
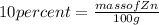
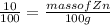
Multiplying both side by 100 g
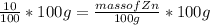
Mass of Zn = 10 g
Step 2: To find mass of Al.
Mass of alloy = (mass of Al + mass of Zn )
100 g = Mass of Al + 10 g
Mass of Al = 90 g
Step 3 : To find moles of both Al and Zn
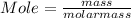
Given : Mass of Zn = 10 g Molar mass of Zn = 65.39 g/mol
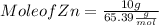
Mole of Zn = 0.153 mol
Given : Mass of Al = 90 g Molar mass of Al = 26.9 g/mol
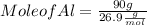
Mole of Al = 3.346 mol
Step 4 : To find number of atoms of Zn and Al .
In 1 mole of any element , the number of atoms present is 6.022 x 10²³ atoms present .
Hence ,
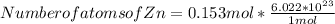
Number of atoms of Zn = 0.921 * 10²³
And ,
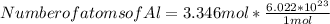
Number of atoms of Al = 20.150 * 10²³
Step 5 : Find atomic fraction of Zn ]
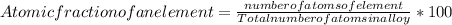
Number of Atoms of Zn = 0.921 * 10²³
Total number of atoms = Atoms of Zn + Atoms of AL
= 0.921 * 10²³ + 20.150 * 10²³ = 21.071 * 10²³
Plugging these values in formula of atomic fraction =>
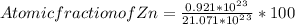
= 0.0437 * 100
Atomic fraction of Zn = 4.37