So we know from the balanced equation that in order for one entire reaction to occur, we need 2 moles of Al and 6 moles of HCl.
We are given 6.0 moles of Al, so let's divide by the amount we need for one reaction:
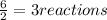
And we are given 13.0 moles of HCl. So let's divide this amount by how much we need for one reaction:
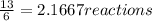
So we know that this amount of HCl will give us less reactions, and therefore it is our limiting reactant. Now we must find the number of moles of
 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.
We know that the molar ratio of HCl to
 is 6:3 in the balanced equation, so we can set up a proportion in order to find this new amount of
is 6:3 in the balanced equation, so we can set up a proportion in order to find this new amount of
 that is produced:
that is produced:
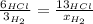
And now we solve for x:
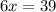
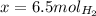
So now we know that 6.5 moles of
 will be produced.
will be produced.
Therefore, our answer is B) HCl is the limiting reactant, 6.5 moles of
 can be formed.
can be formed.