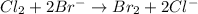Answer: The correct answer is
Step-by-step explanation:
Net ionic equation is defined as the equation in which total number of electrons exchanged are equal.
We are given two half reactions, out of which one is oxidation half reaction and other is reduction half reaction.
Oxidation half reaction:
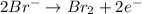
Reduction half reaction:

The net ionic equation for the above reactions will be:
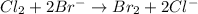
Hence, the correct answer is