The average kinetic energy of an atom in helium gas at temperature T is given by,
K.E=
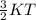
here, K is botzmann constant =1.38×10⁻²³ m² kg s⁻² K⁻¹
Given, K.E= 6.21×10⁻²¹ J
Substituting the values,
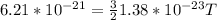
T= 300 K
Therefore, at temperature 300 K the average kinetic energy of the atom in helium gas is 6.21×10⁻²¹ J.