So we know that the formula to calculate molarity is:
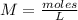
Where M is the molarity, moles is the number of moles in the solution, and L is the volume of the solution in liters.
So we are given the molarity of the solution (5.80 M) and the volume of the solution in liters (6.5 L). So now we just need to plug these numbers in and solve for moles:
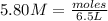
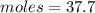
So now we know that there are 37.7 moles of lithium nitrate in the solution.