Answer: The correct answer is a partial negative charge.
Step-by-step explanation:
Water is a molecule which is made by the combination of hydrogen and oxygen atoms.
Hydrogen and oxygen are both non-metals. Thus, the bond between them will be formed by the sharing of electrons and this bond is known as covalent bond.
Oxygen atom is more electronegative than hydrogen atom which means that it will attract the shared pair of electron more towards itself.
Charge on oxygen and hydrogen atom in water molecule:
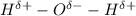
Hence, oxygen atom will carry a partial negative charge due to the formation of covalent bond.