Answer:
0.30 moles of sodium thiosulfate formula units are needed to make 0.10 moles of AgBr soluble.
Step-by-step explanation:
![3Na_2S_2O_3+AgBr\rightarrow NaBr+Na_5[Ag(S_2O_3)_3]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dkn9tmpnvximldfhl11ijcwpv764zk95a3.png)
According to reaction , 1 mole of silver bromide dissolves in 3 moles of Sodium thiosulfate .
Then 0.10 moles of AgBr will dissolve with:
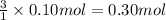
0.30 moles of sodium thiosulfate formula units are needed to make 0.10 moles of AgBr soluble.