Answer:
The 2 mean in the formula
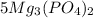 that there 2 phosphates ion in single molecule of magnesium phosphates.
that there 2 phosphates ion in single molecule of magnesium phosphates.
Step-by-step explanation:
Subscript numbers are defined as the number of atoms of each element present in a compound.
Superscript numbers are defined as the charges of ions reacting in a chemical reaction.
The chemical formula of magnesium phosphate is given as:
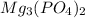
The numeral '2' in the subscript out of the bracket enclosing phosphate ion indicates that there are two phosphate ions in 1 molecule of magnesium phosphate. In which two phosphates ions are linked to single magnesium atom.
And numeral '5' in front of
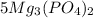 indicates that there are 5 molecules of magnesium phosphate.Numeral written before the molecular formula of any compound in chemical reaction are also termed as coefficient.
indicates that there are 5 molecules of magnesium phosphate.Numeral written before the molecular formula of any compound in chemical reaction are also termed as coefficient.