Answer: The elements present in Group 5A are Nitrogen (N), Phosphorus (P) and Bismuth (Bi).
Step-by-step explanation:
The elements belonging to Group 5A has an electronic configuration
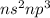 where n is the number of periods.
where n is the number of periods.
For the given options, the electronic configuration of the elements are:
1. Se =
![[Ar]4s^23d^(10)4p^4](https://img.qammunity.org/2019/formulas/chemistry/college/cm2fiunk66ym09n9sj5l9yib6khjzot5cu.png)
2. Te =
![[Kr]5s^24d^(10)5p^4](https://img.qammunity.org/2019/formulas/chemistry/college/ud4vkso00wl8q4x75yhsnhqtdhpp7tln8x.png)
3. Po =
![[Xe]6s^24f^(14)5d^(10)6p^4](https://img.qammunity.org/2019/formulas/chemistry/college/f7rlt1vdig7z7d34e38zia7wap4jyrijpp.png)
4. Rb =
![[Kr]5s^1](https://img.qammunity.org/2019/formulas/chemistry/college/sz983i8zzbf3ceis2jytwtyln8wrmya2cx.png)
5. Xe =
![[Kr]5s^24d^(10)5p^6](https://img.qammunity.org/2019/formulas/chemistry/college/q6yhkzvz82sgok9wmme5wbn0f6w5zcef6h.png)
6. Sn =
![[Kr]5s^24d^(10)5p^2](https://img.qammunity.org/2019/formulas/chemistry/college/obfr0fjzp43o2t60jg11ln5b3avj1ie8bq.png)
7. Pb =
![[Xe]4f^(14)5d^(10)6s^26p^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/6fwba5g0q2uxq25dk289lyt72xnnoi6qql.png)
8. N =
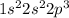
9. P =
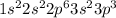
10. Bi =
![[Xe]4f^(14)5d^(10)6s^26p^3](https://img.qammunity.org/2019/formulas/chemistry/college/w75p7ooxprczyc6c99t937cvv4jn9ycei1.png)
From the above configurations, the elements belonging to Group 5A are Nitrogen (N), Phosphorus (P) and Bismuth (Bi).