Answer: Option (d) is the correct answer.
Step-by-step explanation:
According to Avogadro's number, every 1 mole of a substance contains
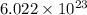 atoms or particles.
atoms or particles.
Now, it is given that there are
 atoms of manganese are present. Hence, calculate its number of moles as follows.
atoms of manganese are present. Hence, calculate its number of moles as follows.
No. of moles =
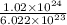
= 1.69 mol
As we know that, number of moles is equal to mass of a substance divided by its molar mass. Since, molar mass of manganese is 54.93 g/mol.
No. of moles =
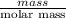
1.69 mol =
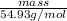
mass = 93 g
Therefore, we can conclude that the mass in grams of
 atoms of manganese (Mn) are
atoms of manganese (Mn) are
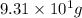 .
.