We need to first find the molar weight of manganese, found on the periodic table. Using a periodic table, we find that manganese has a molar weight of 54.938g/mol.
So in order to find the number of moles present in 165g, we must multiply and cancel out units:
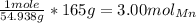
Therefore, we now know that 165g of manganese produces C) 3.00 moles.