We know, 1 mole of any element/compound contains 6.022 × 10²³ atoms/molecules .
Now, we have to calculate the number of molecules in 0.5 mole of sulfur dioxide, SO₂.
So, number of molecules of SO₂ in 0.5 mole is :
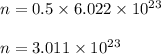
Therefore, number of molecules in 0.5 mole of SO₂ is 3.011 × 10²³.