Step-by-step explanation:
It is known that in an aqueous (liquid) solution the atoms are somewhat closer together as compared to the atoms present in a gas.
So, in an aqueous solution there will be less number of collisions between the atoms.
On the other hand, molecules of a gas are held by weak intermolecular forces. So, they are able to move rapidly from one place to another with more number of collisions.
Hence, we can conclude that the reaction
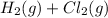 will be faster.
will be faster.