The De Broglie's wavelength of a particle is given by:

where
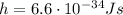 is the Planck constant
is the Planck constant
p is the momentum of the particle
In this problem, the momentum of the electron is equal to the product between its mass and its speed:
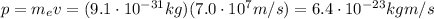
and if we substitute this into the previous equation, we find the De Broglie wavelength of the electron:
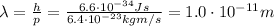
So, the answer is True.