The molar concentration will be greater than 0.01 M
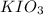 .
.
Since more of the compound was measured out than what was calculated, you can think of the solution as being 'stronger' than what it was calculated to be. Since a 'stronger' concentration results in a number that is higher, the molarity of this solution is going to be greater than 0.01 M.