Molecular mass of C₂H₄ is,
M = 2×12 + 4×1 g/mol
M = 28 g/mol
Moles of C₂H₄ in 5.6 g of C₂H₄ :
n = 5.6/28 mol
n = 0.2 mol
Now, 1 mol of C₂H₄ contains 2 moles of carbon.
So, number of moles of carbon are :
n = 0.4 mol
We know, 1 mol of any atom contains 6.022 × 10²³ atoms.
So, number of carbon atoms are :
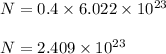
Hence, this is the required solution.