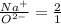Answer:
Ratio represents the relationship between Na and O is 2:1.
Step-by-step explanation:
Given that for every oxygen ion two sodium ions are needed to balance charge.
1 oxygen ion requires 2 sodium atoms
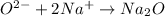
The ratio of oxygen ion to sodium ions is:
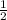
So, on reversing the ratio we will get the ratio of sodium ions to oxygen ions: