Answer : Option B)
![K_(eq) = ([SO_(3)]^(2) )/([SO_(3)]^(2) . [O_(3)]^(2))](https://img.qammunity.org/2019/formulas/chemistry/high-school/yh9d6pmns8cm43j96w8uqblkf418b4gct8.png)
Explanation : The equilibrium constant is often expressed in terms of the molar concentration of products divided by the product of molar concentrations of reactants.
So,
![K_(eq) = ([products])/([reactants])](https://img.qammunity.org/2019/formulas/chemistry/high-school/jrv3mifog4il4ivuqgsb1man8zajfp86vh.png)
.
Now, here in the given reaction of
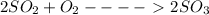
So, we have the equation for equilibrium as
![K_(eq) = ([SO_(3)]^(2) )/([SO_(3)]^(2) . [O_(3)]^(2))](https://img.qammunity.org/2019/formulas/chemistry/high-school/yh9d6pmns8cm43j96w8uqblkf418b4gct8.png)