We can think of 4.70 moles of
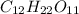 as
as
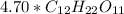 .
.
This means that if we are trying to find the number of moles of hydrogen that are present in this compound, we must multiply 4.70 and 22 to find the total number of moles that are present:
4.70 * 22 = 103.4 moles of hydrogen
So now we know that in 4.70 moles of
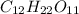 , there are 103.4 moles of hydrogen present.
, there are 103.4 moles of hydrogen present.