When asked to find molar mass, we first need to calculate the total number of atoms of each element in the compound.
In
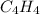
, we see that there are 4 Carbons and 4 Hydrogens.
We then need to look up the atomic mass of each of these elements, which is found on the periodic table.
For carbon, the atomic mass is 12.01g
For hydrogen, the atomic mass is 1.008g
Then we multiply the number of atoms in the element by the atomic mass of the element:
C:
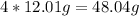
H:
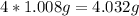
And then we need to add these two values together to get the molar mass of the compound:
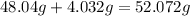
So now we know that
the molar mass of
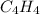 is 52.072g
is 52.072g.