We must first find the molar weight of Tungsten, found on the periodic table as 183.84g per mole.
We are told to find the mass of a sample containing 0.700 moles of Tungsten, so we can set this up in a multiplication equation and cancel units to find the mass in grams:
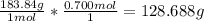
So the answer that is closest to this value is
C) 129g.