Answer 1) In the given reaction of sulfuric acid

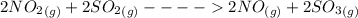
On addition of nitrogen monoxide gas the reaction rate increases and more amount of product is formed.
So, it is clear that NO is the catalyst in this reaction.
Answer 2) This can be proven that NO is catalyst because it increases the rate of the reaction, but it is not consumed during the reaction, and it also gets regenerated at the end of reaction.
Hence, nitrogen mono oxide is considered as the catalyst in the given reaction.
Answer 3) It increases the rate of reaction by decreasing the activation energy of the reaction. Also it can be clearly seen in this reaction the NO is reacting with oxygen to lower the energy of activation. So, it is providing an alternative pathway for proceeding the reaction. This all confirms the assumptions of NO being the catalyst.
