Step-by-step explanation:
According to Boyle's law, at constant temperature and constant number of moles volume will be inversely proportional to pressure applied on a substance.
Mathematically, Volume
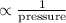
Therefore, it means that decrease in volume will increase the pressure and vice-versa.
Hence, we can conclude that if a gas is moved from a large container to a small container but its temperature and number of moles remain the same, then there will be increase in the pressure of gas.