Answer:
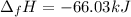
Step-by-step explanation:
Hello,
In this case, by knowing the enthalpy of formation of the copper (II) chloride is -220.1kJ/mol and there are 0.3 moles of such compound, the resulting whole energy is obtained by multiplying those values as the moles define the total energy as shown below:
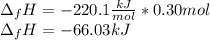
Best regards.