Answer: The correct answer is E and D.
Step-by-step explanation:
 is defined as the difference in the total energy of the products to the total energy of the reactants. It is negative for exothermic reactions and positive for endothermic reactions.
is defined as the difference in the total energy of the products to the total energy of the reactants. It is negative for exothermic reactions and positive for endothermic reactions.
Equation used to represent this quantity follows:
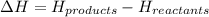
From the given graph,
The starting point of the reaction is represented by the line E, which is the energy of the reactants and the ending point of the reaction is represented by the line D which is the energy of the products.
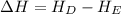
As, the energy of the products is lower than the energy of the reactants. Hence, this reaction is a type of exothermic reactions.
Therefore, the correct answer is E and D.