Answer: 70.2 g
Explanation:
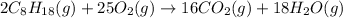
According to the law of conservation of mass, the mass of the products must be same as the mass of the reactants in every chemical equation. Thus the number of atoms of every element must be same on both sides of the equation.
2 moles of octane weigh =
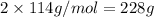
25 moles of oxygen weigh =
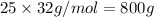
Thus 228 g of octane reacts with 800 g of Oxygen.
20 g of octane reacts with
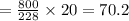 g of Oxygen.
g of Oxygen.