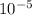Answer : The complete question is attached in answer.
The value of K will be = 2 X
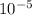
Explanation : We can use the formula as;
∆G = -RT ln K
on rearranging we get, K =
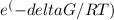
Therefore, we get,
∆G/RT = (26.81 kJ/mol ) / (0.008314 kJ/mol-K) X (298K) = 10.82
So, K =
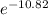
Therefore, K = 2 X