Answer : The mole fraction of nitrogen will be 0.4615.
Explanation : When nitrogen (
 )and hydrogen (
)and hydrogen (
 )are mixed, the mole ratio becomes 1 : 1.5,
)are mixed, the mole ratio becomes 1 : 1.5,
Now we know that (
 ) is acting as a limiting agent.
) is acting as a limiting agent.
So at the time of when 0.4 moles of (
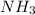 ) is been formed it requires 0.4 moles of (
) is been formed it requires 0.4 moles of (
 ) and 3.4 moles of (
) and 3.4 moles of (
 )
)
So, we find the the remaining (
 ) will be 0.6 and
) will be 0.6 and
(
 ) will be 0.3 mole present in mixture.
) will be 0.3 mole present in mixture.
So, the mole fraction of (
 ) becomes = 0.6 / (0.6 + 0.4 + 0.3) Which becomes = 0.4615
) becomes = 0.6 / (0.6 + 0.4 + 0.3) Which becomes = 0.4615