Answer : The mass of the sample of NaCl will be, 1417.7 grams.
Explanation : Given,
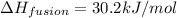
This means that,
As, 1 mole of sample of NaCl has an enthalpy of fusion = 30.2 kJ
And, 1 mole of NaCl has the mass = 58.44 grams
As, 30.2 kJ of heat is needed for a mass 58.44 grams of NaCl
So, 732.6 kJ of heat will be needed for =
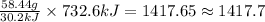 grams of NaCl.
grams of NaCl.
Hence, the mass of sample of NaCl will be, 1417.7 grams.