Answer : We can produce 125.7 g of
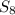 .
.
Explanation : The reaction will be

The molecular mass of
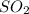 is 64.1 g/mol
is 64.1 g/mol
and molecular mass of
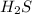 is 34.1 g/mol
is 34.1 g/mol
For every mole of
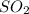 we would need twice of
we would need twice of
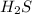 moles, so for every 3 moles of
moles, so for every 3 moles of
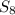 we need 16 moles of
we need 16 moles of
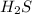
Now, we can calculate number of moles
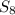
2.61 X (3/16) = 0.49 moles
Here, the molecular mass of
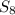 is 256.8 g
is 256.8 g
multiplying it with the number of 0.49 moles we get, 256.8 X 0.49 = 125.7 g of
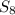 .
.
Hence, 125.7 g of
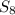 will be produced.
will be produced.