Step-by-step explanation:
It is known that the relation between kinetic energy and temperature is as follows.
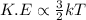
Therefore, when there will be increase in temperature then there will also occur increase in kinetic energy of the particles of a substance.
Hence, when temperature of a solid substance is increased then its molecules will gain kinetic energy leading to an increase in the number of collisions.
As a result, molecules will start to move away from each other.
Therefore, solid substance will successively convert into liquid substance.