Silver carbonate dissociates and show equilibrium as shown below
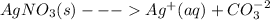
so there is formation of carbonate ions
When we add strong acid nitric acid in it , it gives proton (hydronium ion)
These
 reacts with carbonate ions and thus decreases the concentration of carbonate ions in the solution. Due to decrease in concentration products the system will shift in the direction where there can be an increase in concentration of products. Hence system or equilibrium shifts in forward direction. This will cause more solubility of silver carbonate Or we can say that the silver carbonate dissolves
reacts with carbonate ions and thus decreases the concentration of carbonate ions in the solution. Due to decrease in concentration products the system will shift in the direction where there can be an increase in concentration of products. Hence system or equilibrium shifts in forward direction. This will cause more solubility of silver carbonate Or we can say that the silver carbonate dissolves