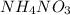Answer : BaS
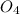 will be the precipitate which will be formed.
will be the precipitate which will be formed.
Explanation : When all the three solutions namely;
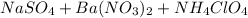 are mixed together a white precipitate of BaS
are mixed together a white precipitate of BaS
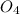 is formed as a product in the solution along with the soluble by product of Ammonium nitrate which is
is formed as a product in the solution along with the soluble by product of Ammonium nitrate which is