Answer : 5100 KJ
Explanation : In the given reaction of
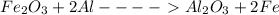
we can see that 2 moles of Al is producing -850 KJ of energy
so for 12 moles of Al we can calculate as;
12 moles of Al X

= -
5100 KJ
So, we can say that 12 moles of Al will need -5100 KJ of energy.