Answer :
Option C) Reaction proceeds to the left towards the reactants.Explanation : When we know the equilibrium constant (K)is given we can simply compare it with the reaction quotient (Q) and find which direction the reaction would proceed to establish an equilibrium state.
So, in the given reaction of 2NOCl ----> 2NO +
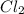
Here the concentration of each molecule is given we can use them in following reaction;
![Q = ([ NO]^(2). [Cl_(2) ])/([ NOCl ]^(2))](https://img.qammunity.org/2019/formulas/chemistry/high-school/4b9yzgrc1tdolyc4a68uvinht3y0ntovyt.png)
on substituting the values weget Q = 0.125
on comparing with K which is 4.4 X
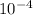
we get
Q > K so the reaction proceeds to form the NOCl to increase the concentration of NOCl and establish an equlibrium.