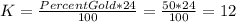Answer:
Karat number for the alloy = 12
Step-by-step explanation:
Given:
Mol% gold = 50
Mol% silver = 50
To determine:
The karat number of the alloy
Step-by-step explanation:
A Karat number is a parameter which is used to describe alloys containing gold based on the % gold in the alloy.
The corresponding formula is:
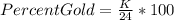
where K= Karat number