Answer :
The enthalpy per mole for combustion of
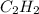 is -1255.6 KJ/mol
is -1255.6 KJ/mol
Explanation : To find the enthalpy of combustion we need to use the formula as Δ H =
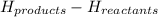
The reaction is
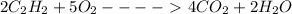
On substituting the values in the above equation; we get;
(4 X -393.5) + ((2 X 241.82) - (2 X 226.77) - (5 X 0) = - 2511.2 KJ/ mol
The enthalpy here we get is for 2 moles of
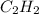
which is -2511.2 KJ/mol
we need for one mole of
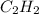
will be -2511.2 / 2 =
- 1255.6 KJ/mol