Step-by-step explanation:
A oxidizing agent or oxidizer is a substance that loses electrons and itself gets reduced.
Also, a substance or element that has more positive value of
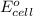 or reduction potential will act as a strong oxidizer because it will easily get reduced.
or reduction potential will act as a strong oxidizer because it will easily get reduced.
Therefore, standard reduction potential of the given elements is as follows.
K = -2.92
Ag = 1.83
Au = 0.80
Cu = 0.16
Ni = -0.23
Ca = -2.76
Hence, Ag has the highest positive value and calcium has the lowest. Thus, list from strongest to weakest oxidizer are as follows.
Ag > Au > Cu > Ni > Ca > K