Answer :
4514.311 torr
Explanation : Using henry's law in simplified version;

where
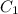
is the concentration of gas in first case which is 0.0225 g
and
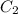
is the concentration of gas in second case which is 0.134 g
the concentration of water is ignored as it remains the same in both the cases.
And,
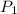
is 758 torr and
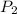
needs to be found.
on substituting the values we get,
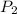
= (0.134 X 758) / 0.0225 =
4514.311 torr