Answer: There is no effect of catalyst on a system in equilibrium.
Step-by-step explanation: A equilibrium reaction is one in which rate of forward reaction is equal to the rate of backward reaction.
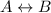
Addition of a catalyst to a equilibrium reaction affects the forward and backward reactions equally and thus do not affect the equilibrium.