Answer:- 29.6 moles of carbon.
Solution:- We have been given with 3.7 moles of
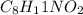 and asked to calculate the moles of C.
and asked to calculate the moles of C.
Looking at the formula of the compound, there are 8 carbons in it means 1 mol of he compound has 8 moles of C. So, if we multiply the given moles of the compound by 8 then we get the moles of C.
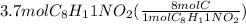
= 29.6 mol C
Hence. there are 29.6 moles of C in 3.7 moles of
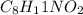 .
.