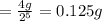Answer :
0.125 g
Explanation : If you are calculating the half life of P-32 which has half life of 14.3 days and you want to calculate how many grams are remaining after 71.3 days if yo are starting with 4 g?
starting you can calculate the number of half life P-32 undergoes first,
which is n = 71.5 / 14.3 = 5 days.
now, to calculate the remaining mass,
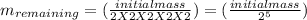
=