Answer : To find the molar mass of unknown gas we need to solve the equation of rate of diffusion and molecular weight of the gases, which is as follows;
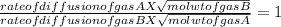
here the mol wt of gas B is not known so we take it as '
x'
on solving we get 7 X
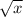
/ 4.9 X
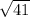
mol. wt of unknown gas =
20.0 g